MONAGHAN MEDICAL WINS 2024 AARC ZENITH AWARD
Respiratory Care Leader Honored for Excellence in Products and Services
[PLATTSBURGH, NY | October 3, 2024 – Monaghan Medical Corp., an innovator in the development and manufacturing of respiratory devices, proudly announces its recognition as a recipient of the prestigious 2024 Zenith Award, presented by the American Association for Respiratory Care (AARC). This accolade reaffirms Monaghan Medical’s commitment to excellence in the field of respiratory care.
The 2024 AARC Zenith Award recognizes outstanding contributions in the respiratory care profession. Monaghan was recognized by AARC members for their exceptional products, accessible sales staff, responsiveness, service record, truth in advertising, and unwavering support of the respiratory care profession.
“The AARC Zenith Award is the ‘people’s choice’ award of the respiratory care profession, and we are honored to have earned this distinction,” said Bill Seitz, President at Monaghan Medical. “Our commitment to providing top-quality products and support to respiratory therapists has been recognized by those at the forefront of patient care, and we are deeply grateful.”
The award will be formally presented to Monaghan Medical at AARC’s International Respiratory Congress in Orlando, Florida. With more than 47,000 members, AARC is the leading national and international professional association for respiratory care professionals and allied health practitioners.
FOR MORE INFORMATION
Dominic P. Coppolo, Senior Vice President of Clinical and Hospital Marketing
Monaghan Medical Corp.
Phone: 800-343-9071
Email: dcoppolo@monaghanmed.com
Website: www.monaghanmed.com

William T. Seitz, MBA
President, Monaghan Medical Corporation
ABOUT MONAGHAN MEDICAL
Headquartered in Plattsburgh, New York, Monaghan Medical is a leader in the research, development, manufacture, and marketing of respiratory devices to manage acute and chronic pulmonary illnesses and diseases like asthma and chronic obstructive pulmonary disease (COPD). MMC’s mission is to provide the respiratory community with quality, researched, and innovative devices to help people breathe easier.
MONAGHAN MEDICAL CORPORATION ATTAINS ISO 14001 CERTIFICATION FOR ENVIRONMENTAL MANAGEMENT EXCELLENCE
PLATTSBURGH, NY, FEBRUARY 27, 2024 – Monaghan Medical Corporation (MMC) is proud to have achieved ISO 14001 certification, underscoring the company’s dedication to continually improving environmental management practices.
ISO 14001 is an internationally recognized standard for organizations to design and implement an effective Environmental Management System (EMS). MMC’s commitment to the ISO standard assures environmental risks are systematically managed and continuously improved. Risk-based thinking and the Plan-Do-Check-Act (PDCA) approach, many key themes within ISO 14001, are similar to other ISO standards, such as ISO 13485, a standard MMC has attained in the past. This certification serves as a differentiator, assuring customers of MMC’s commitment to environmental compliance and stewardship.
“This certification signifies MMC’s unwavering commitment to taking care of the environment, while continuing on our mission to deliver innovative medical devices that help people breathe better… We are proud to implement rigorous standards that go beyond compliance, demonstrating our dedication to sustainability through reducing greenhouse gas (GHG) emissions and waste from our operations.” – Andrew Sepcie, Vice President of Operations at MMC.
MMC’s Environmental Management System (EMS) outlines key objectives for sustainable practices, aiming to improve our waste diversion rate with a proactive approach to recycling and waste reduction. Simultaneously, the company has set a longer-term goal to make significant reductions in our greenhouse gas (GHG) emissions.
ABOUT MONAGHAN MEDICAL CORPORATION
Headquartered in Plattsburgh, New York, Monaghan Medical Corporation is a leader in the research, development, manufacture, and marketing of respiratory devices to manage acute and chronic pulmonary illnesses and disease processes like asthma and chronic obstructive pulmonary disease (COPD). MMC’s mission is to provide the respiratory community with quality, researched, and innovative devices to help people breathe easier.

Monaghan Medical Corporation manufacturing headquarters in Plattsburgh, New York
Flovent Inhaler Discontinued! Here’s what you need to know!
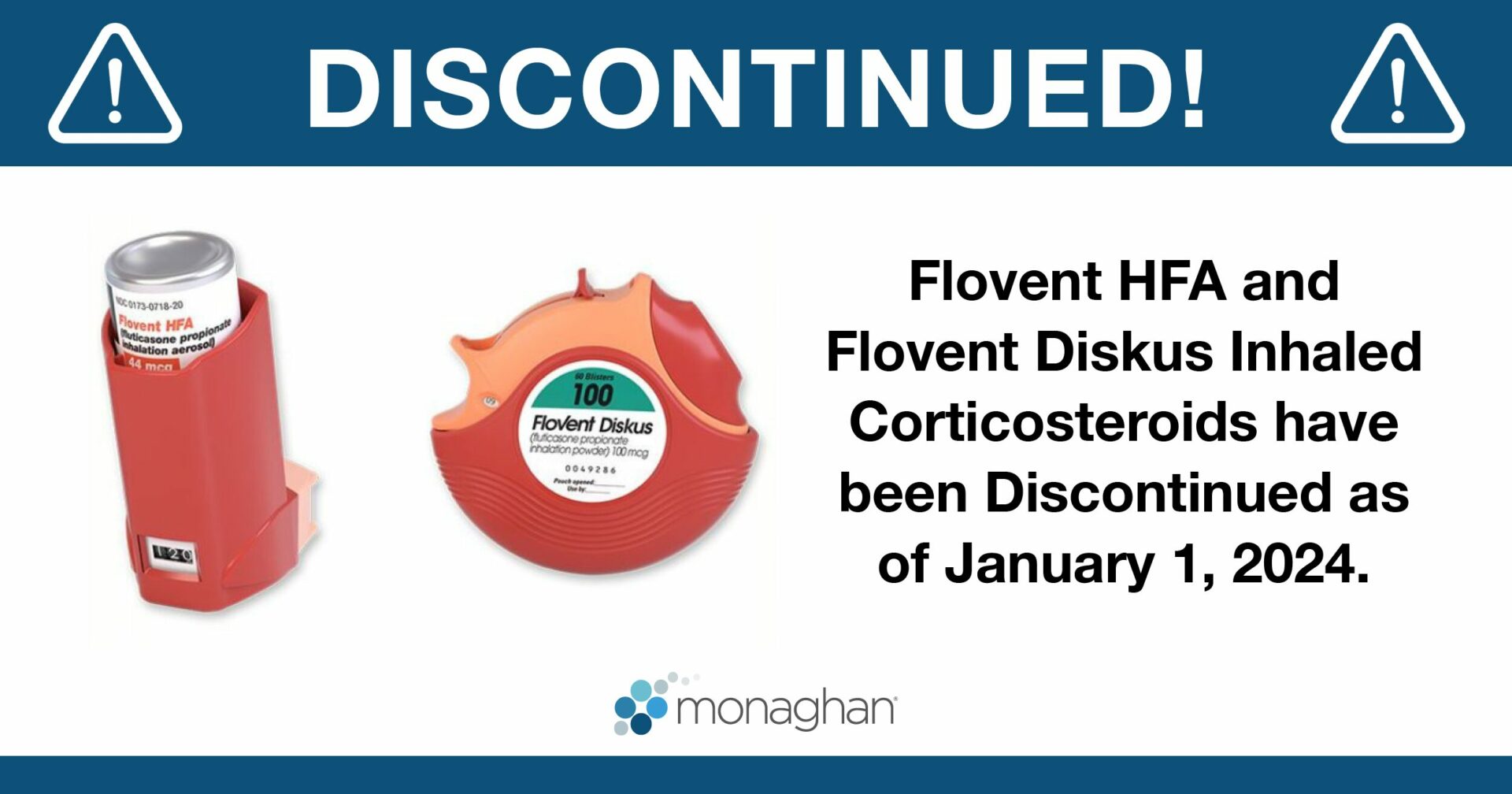
FACTS AT A GLANCE:
-
On January 1, 2024, GSK discontinued the production of both Flovent HFA MDI and Diskus DPI formulations, replacing them with generic versions distributed by Prasco LLC.
-
Generic Fluticasone may lead to higher copayments for patients or might not be covered by insurance.
-
Valved Holding Chambers (VHCs), like the AeroChamber® Brand, address timing issues often associated with using an MDI alone.
-
Using the AeroChamber® Plus Flow-Vu aVHC with other inhaled corticosteroids on the market as a substitute for Flovent shows improved medication delivery and reduced oropharyngeal delivery.
Flovent, a popular corticosteroid inhaler used to treat and control asthma symptoms in children and adults, has been discontinued. As of January 1, 2024, GlaxoSmithKline (GSK) discontinued the production of Flovent HFA and Flovent Diskus, replacing them with a generic version of each device at the same previous strengths.1 In a statement to the Asthma and Allergy Foundation of America (AAFA), GSK stated, “The authorized generic will contain the same medicine, in the same familiar device, with the same instructions for use as Flovent.”1
GSK claims this transition aims to provide patients in the U.S. with potentially lower-cost alternatives to these medically important products.1 However, some medical professionals express concerns about potential impacts on patients’ health and finances.
MEDICAL PROFESSIONALS’ CONCERNS:
The American Academy of Pediatrics has expressed concerns and warns of higher copayments and delayed access due to authorization requirements.2 Additionally, pediatricians worry about alterations to the drug’s delivery mechanism, as some insurers only cover breath-actuated inhalers, which may not be suitable for children with specific asthma conditions.2 Metered dose inhalers (MDIs) can be used by children and adults alike, and improved medication delivery can be recognized by the addition of a valved holding chamber.3
AEROCHAMBER PLUS® FLOW-VU® aVHC:
The AEROCHAMBER PLUS® FLOW-VU® Anti-Static Valved Holding Chamber (aVHC) has been designed to deliver the intended dose of medication established by pharmaceutical companies.4 These devices contain a one-way valve to hold the medication emitted from the MDI, making it available to inhale when the user breathes in through the chamber. The AEROCHAMBER PLUS® Brand of Valved Holding Chambers:
- Reduces the need to coordinate the inhaled breath with MDI activation.3
- Reduces oral medication deposition that can cause systemic side effects.3
- Improves lower respiratory tract deposition.3
Data shows improved medication delivery and decreased oropharyngeal delivery using an AEROCHAMBER PLUS® FLOW-VU® aVHC with other inhaled MDI corticosteroids such as Alvesco5 and QVar.6
In uncertain times of medication transitions, the AEROCHAMBER PLUS® brand of aVHC remains a reliable and consistent choice for effective inhalation therapy. Available in your local pharmacy with a prescription.
For any questions or concerns, make sure to discuss your options with your healthcare provider and ensure the best outcomes for your respiratory health.
SOURCES:
- Asthma and Allergy Foundation of America (AAFA). (2024, Jan 4). Flovent HFA and Flovent Diskus Asthma Medicines Being Discontinued. Retrieved on January 10, 2024.
- Jenco, M. (2023 Dec 8). Experts call on insurers to prioritize corticosteroid medicines appropriate for children. Retrieved on January 12, 2024.
- McIvor, R. Devlin, H. Kaplan, A. (2018). Optimizing the delivery of inhaled medication for respiratory patients: The role of valved holding chambers. Canadian Respiratory Journal 2018:5076259. Retrieved on January 31, 2024.
- Harkness H, Doyle C, Ali R, Avvakoumova V, Mitchell J, Nagel M. Valved Holding Chambers are non-interchangeable: Development of a Universal VHC that provides assurance of drug delivery to patients and healthcare providers. Presented at 2012 Canadian Respiratory Conference.
- Nagel, M. Suggett, J. (2022). Assessment of potential oropharyngeal deposition and lung delivery of suspension and solution formulated inhaled corticosteroid formulations delivered by pressurized metered dose inhaler without and with a spacer using an anatomic upper airway. Poster presented at the Canadian Respiratory Conference, April 7-9, in Victoria BC.
- Suggett, J. Nagel, M. Mitchell, J. (2017). Assessment of potential mouth/throat deposition and lung delivery of suspension- and solution-formulated inhaled corticosteroid formulations delivered by pressurized metered dose inhaler without and with a valved holding chamber using an anatomic adult upper airway. Poster presented at Drug Delivery to the Lungs (DDL), December 6-8, 2017, in Edinburgh Scotland.
LEARN MORE ABOUT AEROCHAMBER PLUS® FLOW-VU® aVHC:
-

AEROCHAMBER PLUS® FLOW-VU® AVHC with Large Mask
Rated 0 out of 5 View More -

AEROCHAMBER PLUS® FLOW-VU® AVHC with Medium Mask
Rated 0 out of 5 View More -

AEROCHAMBER PLUS® FLOW-VU® AVHC with Small Mask
Rated 0 out of 5 View More -

AEROCHAMBER PLUS® FLOW-VU® AVHC with Mouthpiece
Rated 0 out of 5 View More
Benefits of using an AEROCHAMBER PLUS® FLOW-VU® Valved holding Chamber with your inhaler.
Chambers help inhaler medications reach your lungs where they are needed most and limit the amount that ends up wasted in the back of your throat.
Individuals who use the AEROCHAMBER PLUS® FLOW-VU® chamber with their inhaler experience better control of their respiratory symptoms.
To enhance drug delivery, respiratory guidelines recommend the use of chambers when using inhalers for people of all ages.
https://youtu.be/n_8ySTIib4Y?si=CMO8vQN475YzsdRv
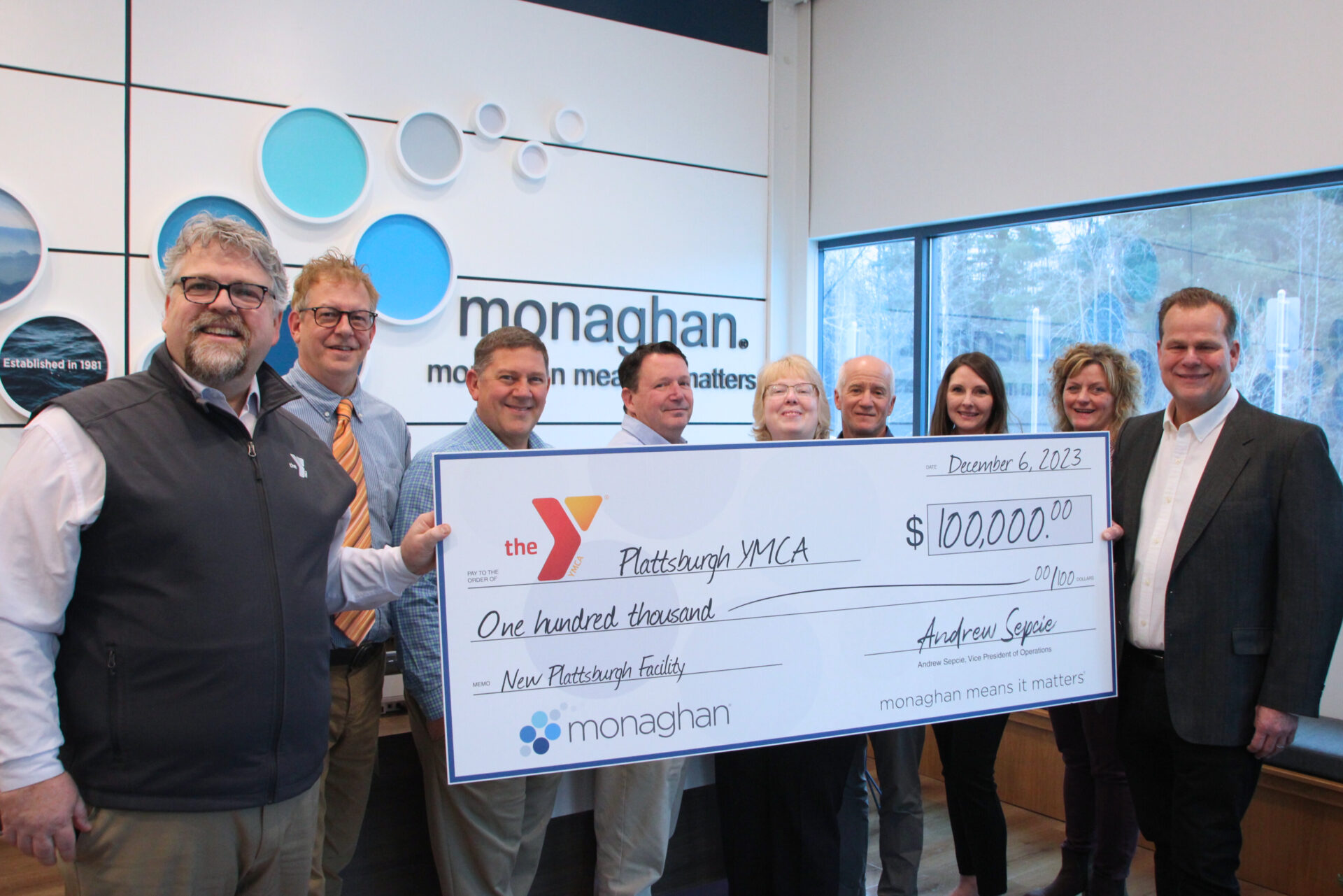
Monaghan Medical Corporation Demonstrates Commitment to Plattsburgh Community With $100,000 Donation for New YMCA
PLATTSBURGH, NY, December 7, 2023 –
Monaghan Medical Corporation (MMC), a leading player in the respiratory device industry, is proud to announce a $100,000 donation in support of the Plattsburgh community. The contribution will be dedicated to the construction of a new YMCA in Plattsburgh, reinforcing MMC’s commitment to the well-being and vitality of the local community.
The cornerstone of this philanthropic endeavor is the naming of the Main Entrance Pagoda at the new YMCA in honor of Monaghan Medical Corporation. This tangible symbol of Monaghan’s dedication underscores its commitment to fostering a healthy and active lifestyle within the Plattsburgh community.
Andrew Sepcie, Vice-President of Operations at Monaghan Medical, emphasized their commitment, stating, “Monaghan is proud to be part of the YMCA project, creating a positive impact on the lives of thousands of people and investing in the future of our community.”
Justin Ihne, CEO of the Plattsburgh YMCA, expressed gratitude, stating, “We are humbled and grateful to Monaghan Medical for this amazing gift. This project is going to transform health and wellness in the North Country, and we’re thrilled that Monaghan Medical is a part of that.”
Moreover, the YMCA has received an anonymous offer to match any donations made in October up to $100,000, effectively doubling the impact of Monaghan Medical Corporation’s generous contribution. This means that the YMCA will receive an additional $100,000 from this generous match, further enhancing the positive influence on the Plattsburgh community.
 Monaghan Medical Corporation Partners with Plattsburgh Community: $100,000 Donation Sparks Vitality in New YMCA Project with Doubling Effect through Anonymous Matching Offer
Monaghan Medical Corporation Partners with Plattsburgh Community: $100,000 Donation Sparks Vitality in New YMCA Project with Doubling Effect through Anonymous Matching Offer
For more information about Monaghan Medical products, please visit www.monaghanmed.com
# # #
ABOUT MONAGHAN MEDICAL CORPORATION
Headquartered in Plattsburgh, New York, Monaghan Medical Corporation is a leader in the research, development, manufacture, and marketing of respiratory devices to manage acute and chronic pulmonary illnesses and disease processes like asthma and chronic obstructive pulmonary disease (COPD). MMC’s mission is to provide the respiratory community with the finest, most researched, and most innovative devices to help people breathe easier.
ABOUT THE PLATTSBURGH YMCA:
The YMCA is for Youth Development, Healthy Living, and Social Responsibility and operates several locations: Two membership locations in Plattsburgh and one in Malone; 400-acre Camp Jericho located in Altona, New York; Camp Tapawingo in Point Au Roche; and Bright Beginnings Child Care Center in Plattsburgh. The Plattsburgh YMCA serves over 8000 families and provides child care and camping locations in Plattsburgh, Peru, Beekmantown, Malone, and Saranac Lake. Youth Sports programs serve over 1400 children annually. For more information, please call 518-561-4290 or email at info@plattsburghymca.org
For more information please contact:
Andrew Sepcie, Vice-President of Operations
Monaghan Medical Corporation
Phone: 800-343-9071
Email: asepcie@monaghanmed.com
Website: monaghanmed.com
Benefits of using an AEROCHAMBER PLUS® FLOW-VU® Valved holding Chamber with your inhaler.
Chambers help inhaler medications reach your lungs where they are needed most and limit the amount that ends up wasted in the back of your throat.
Individuals who use the AEROCHAMBER PLUS® FLOW-VU® chamber with their inhaler experience better control of their respiratory symptoms.
To enhance drug delivery, respiratory guidelines recommend the use of chambers when using inhalers for people of all ages.
https://youtu.be/n_8ySTIib4Y?si=CMO8vQN475YzsdRv