FACTS AT A GLANCE:
-
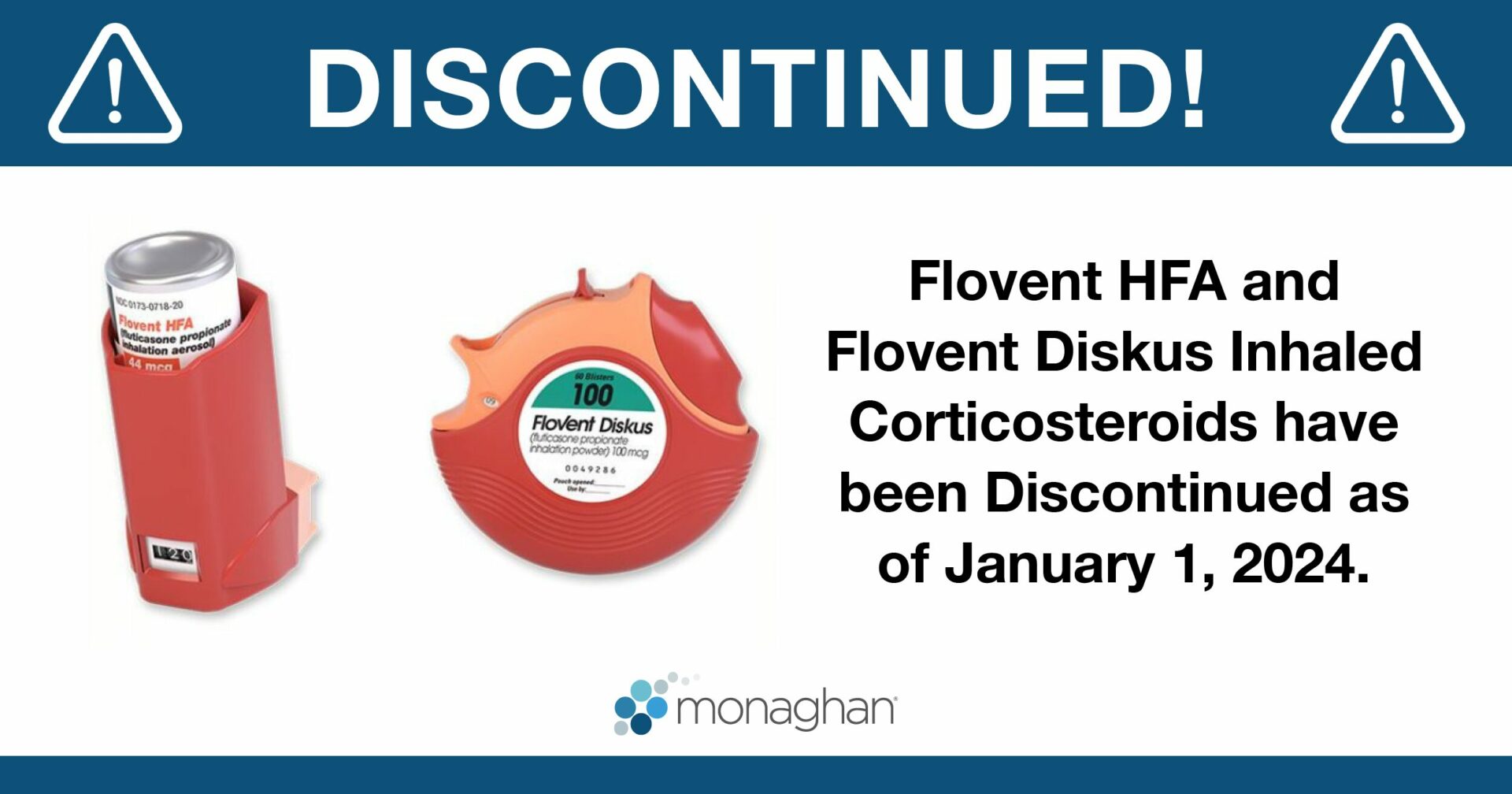
On January 1, 2024, GSK discontinued the production of both Flovent HFA MDI and Diskus DPI formulations, replacing them with generic versions distributed by Prasco LLC.
-
Generic Fluticasone may lead to higher copayments for patients or might not be covered by insurance.
-
Valved Holding Chambers (VHCs), like the AeroChamber® Brand, address timing issues often associated with using an MDI alone.
-
Using the AeroChamber® Plus Flow-Vu aVHC with other inhaled corticosteroids on the market as a substitute for Flovent shows improved medication delivery and reduced oropharyngeal delivery.
Flovent, a popular corticosteroid inhaler used to treat and control asthma symptoms in children and adults, has been discontinued. As of January 1, 2024, GlaxoSmithKline (GSK) discontinued the production of Flovent HFA and Flovent Diskus, replacing them with a generic version of each device at the same previous strengths.1 In a statement to the Asthma and Allergy Foundation of America (AAFA), GSK stated, “The authorized generic will contain the same medicine, in the same familiar device, with the same instructions for use as Flovent.”1
GSK claims this transition aims to provide patients in the U.S. with potentially lower-cost alternatives to these medically important products.1 However, some medical professionals express concerns about potential impacts on patients’ health and finances.
MEDICAL PROFESSIONALS’ CONCERNS:
The American Academy of Pediatrics has expressed concerns and warns of higher copayments and delayed access due to authorization requirements.2 Additionally, pediatricians worry about alterations to the drug’s delivery mechanism, as some insurers only cover breath-actuated inhalers, which may not be suitable for children with specific asthma conditions.2 Metered dose inhalers (MDIs) can be used by children and adults alike, and improved medication delivery can be recognized by the addition of a valved holding chamber.3
AEROCHAMBER PLUS® FLOW-VU® aVHC:
The AEROCHAMBER PLUS® FLOW-VU® Anti-Static Valved Holding Chamber (aVHC) has been designed to deliver the intended dose of medication established by pharmaceutical companies.4 These devices contain a one-way valve to hold the medication emitted from the MDI, making it available to inhale when the user breathes in through the chamber. The AEROCHAMBER PLUS® Brand of Valved Holding Chambers:
- Reduces the need to coordinate the inhaled breath with MDI activation.3
- Reduces oral medication deposition that can cause systemic side effects.3
- Improves lower respiratory tract deposition.3
Data shows improved medication delivery and decreased oropharyngeal delivery using an AEROCHAMBER PLUS® FLOW-VU® aVHC with other inhaled MDI corticosteroids such as Alvesco5 and QVar.6
In uncertain times of medication transitions, the AEROCHAMBER PLUS® brand of aVHC remains a reliable and consistent choice for effective inhalation therapy. Available in your local pharmacy with a prescription.
For any questions or concerns, make sure to discuss your options with your healthcare provider and ensure the best outcomes for your respiratory health.
SOURCES:
- Asthma and Allergy Foundation of America (AAFA). (2024, Jan 4). Flovent HFA and Flovent Diskus Asthma Medicines Being Discontinued. Retrieved on January 10, 2024.
- Jenco, M. (2023 Dec 8). Experts call on insurers to prioritize corticosteroid medicines appropriate for children. Retrieved on January 12, 2024.
- McIvor, R. Devlin, H. Kaplan, A. (2018). Optimizing the delivery of inhaled medication for respiratory patients: The role of valved holding chambers. Canadian Respiratory Journal 2018:5076259. Retrieved on January 31, 2024.
- Harkness H, Doyle C, Ali R, Avvakoumova V, Mitchell J, Nagel M. Valved Holding Chambers are non-interchangeable: Development of a Universal VHC that provides assurance of drug delivery to patients and healthcare providers. Presented at 2012 Canadian Respiratory Conference.
- Nagel, M. Suggett, J. (2022). Assessment of potential oropharyngeal deposition and lung delivery of suspension and solution formulated inhaled corticosteroid formulations delivered by pressurized metered dose inhaler without and with a spacer using an anatomic upper airway. Poster presented at the Canadian Respiratory Conference, April 7-9, in Victoria BC.
- Suggett, J. Nagel, M. Mitchell, J. (2017). Assessment of potential mouth/throat deposition and lung delivery of suspension- and solution-formulated inhaled corticosteroid formulations delivered by pressurized metered dose inhaler without and with a valved holding chamber using an anatomic adult upper airway. Poster presented at Drug Delivery to the Lungs (DDL), December 6-8, 2017, in Edinburgh Scotland.
LEARN MORE ABOUT AEROCHAMBER PLUS® FLOW-VU® aVHC:
-

AEROCHAMBER PLUS® FLOW-VU® AVHC with Large Mask
Rated 0 out of 5 View More -

AEROCHAMBER PLUS® FLOW-VU® AVHC with Medium Mask
Rated 0 out of 5 View More -

AEROCHAMBER PLUS® FLOW-VU® AVHC with Small Mask
Rated 0 out of 5 View More -

AEROCHAMBER PLUS® FLOW-VU® AVHC with Mouthpiece
Rated 0 out of 5 View More
Benefits of using an AEROCHAMBER PLUS® FLOW-VU® Valved holding Chamber with your inhaler.
Chambers help inhaler medications reach your lungs where they are needed most and limit the amount that ends up wasted in the back of your throat.
Individuals who use the AEROCHAMBER PLUS® FLOW-VU® chamber with their inhaler experience better control of their respiratory symptoms.
To enhance drug delivery, respiratory guidelines recommend the use of chambers when using inhalers for people of all ages.
https://youtu.be/n_8ySTIib4Y?si=CMO8vQN475YzsdRv