The Filtered Mouthpiece Kit from Monaghan Medical
The Filtered Mouthpiece Kit helps ensure safe and effective respiratory treatments and is the ideal companion for the AEROECLIPSE® BAN® Nebulizer.
- 510(k) cleared to be safe and effective with the AEROECLIPSE® BAN® Nebulizers (2023).
- Filters exhaled emissions, viruses (99.997%), bacteria (99.9997%), and undelivered medication, reducing exposure to others in the room.2
- Direct inhalation pathway so there’s no loss of medication for the patient, ensuring optimal delivery.
- Tamper-resistant one-way valve adds an extra layer of security.
- Slip-fits easily to the nebulizer and eliminates the need for unnecessary adapters.
- Single-patient, disposable device for use up to 7 days with replacement filters.
The filter kit with the AEROECLIPSE® BAN® Nebulizer – where safety and efficiency meet seamlessly.
Hospital and DME Only:
Not available through retail pharmacies. For provider use only.
BEFORE EACH USE:
Replace immediately if your filter has become damaged or has missing parts.
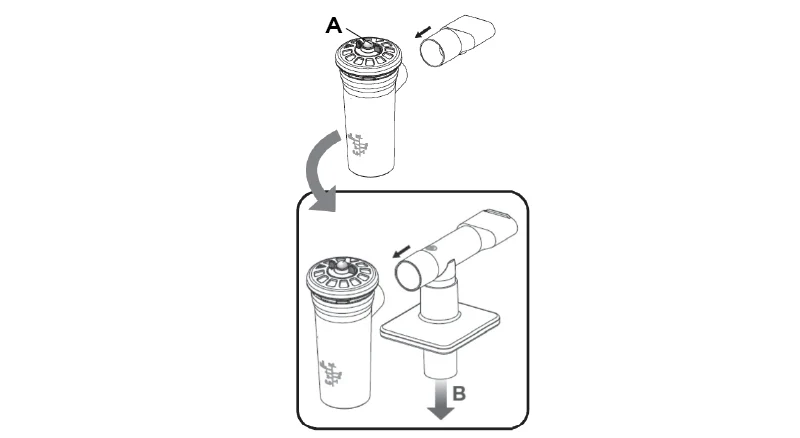
- Replace the mouthpiece of the AEROECLIPSE® BAN® Nebulizer with the assembled filter kit.
- Tightly seal lips around the mouthpiece, and inhale and exhale normally through the device.
- Ensure the filter kit’s exhalation flow path is not blocked.
- Continue treatment using the instructions supplied with the AEROECLIPSE® BAN® Nebulizer.
These are shortened user instructions. Always review the complete instructions that came with your device.
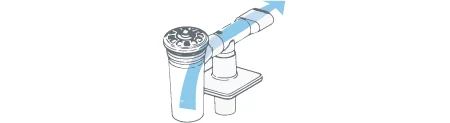
On Inhalation:
- Aerosol is drawn directly from the nebulizer through the tee. The direct inhalation pathway ensures no loss of medication for the patient.1
- There is a one-way valve in the tee which remains closed during inhalation.
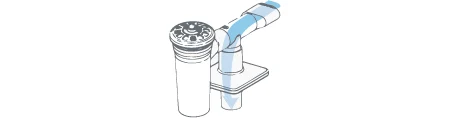
On Exhalation:
Exhaled breath takes the path of lease resistance, opening the one-way valve in the tee and exiting through the filter media.
Frequently Asked Questions
WHY IS 510(K) CLEARANCE IMPORTANT FOR A NEBULIZER AND FILTER KIT COMBINATION?
510(k) clearance validates that a device (or combination of two devices) is safe and effective for its intended use. This is required to ensure that performance of the nebulizer is not impacted with the addition of the filtered mouthpiece kit – for both aerosol delivery and exhalation resistance.
CAN THE FILTER KIT BE USED WITH A FACEMASK?
No, this is for mouthpiece delivery only. Aerosol masks have openings that would allow emissions to escape into the room even with a filter inline (negating the benefit of adding in a filter).
HOW OFTEN CAN I USE THE FILTER BEFORE REPLACING AND WHAT IS THE RECOMMENDED CHANGE OUT GUIDANCE FOR USE?
The Filter Kit is a single patient, disposable device – the mouthpiece is approved for 7 days with single use replacement filters.
It is important to note that this filter can be used for many purposes other than aerosol delivery and thus the above single use wording is necessary. Following the hospital/organizational guidelines for filter replacement, when used with nebulizer therapy, is acceptable. Filters that are damaged or saturated should be changed out immediately.
WHAT ARE THE SPECS OF THE FILTER?
The filter has been tested to filter viral particles to 99.997% and bacterial particles to 99.9997%.
The bacterial and viral filtration efficiency performance of the filtration media has been tested by the material’s manufacturer. Report available upon request.
It is important to note that filters have different ratings for efficiency and the particle sizes that they can filter. Not every filter will function the same even if they look similar. (see the following question/answer: Can any filter be used on the mouthpiece kit?).
CAN THE FILTER MEDIA BE REPLACED?
No, the filter assembly is a single use device and is a sealed unit-not intended to be taken apart. Replacements filters can be ordered separately.
CAN ANY FILTER BE USED ON THE MOUTHPIECE KIT?
The filtered mouthpiece is 510(k) cleared for use with the AEROECLIPSE® BAN™ Nebulizer, but also has standard ISO fittings in which a variety of filters could be used. It is important that a replacement filter have the same specifications as the current Monaghan filter at filtering out the viral and bacterial particles to insure similar functionality. Many filters are on the market and may have different efficiency percentages/ratings (or how much particle and the size they can filter).
WHAT IS THE RECOMMENDED PROCESS IF THE FILTER BECOMES WET OR SATURATED (E.G. SPILLED MEDICATION OR CONTINUOUS OUTPUT NEBULIZATION FOR PROLONGED PERIODS)?
Saturated filters should be changed out immediately or as soon as possible to insure the same high percentage of viral and bacterial filtering. Not only could this have the potential to increase the resistance in the system, the filtering capacity is greatly reduced when the filter media becomes wet or has condensate depositing on the surface. Humidity is OK, but if droplets or pools of moisture are forming on either side of the filter media it should be changed out as soon as possible.
CAN I PLACE TWO FILTERS IN TANDEM TO INCREASE THE EFFICIENCY OF THE FILTERED MOUTHPIECE KIT?
Yes, but you may increase the resistance in the system making it more difficult for the patient to exhale through the nebulizer and filter system. The patient should be monitored closely if using the two filters together for increasing work of breathing and fatigue.
CAN THIS BE USED WITH OTHER NEBULIZERS?
Due to the design of this mouthpiece filter kit, it may be difficult to adapt to other nebulizers.
ARE THERE STUDIES FOR YOUR FILTER KIT?
Yes. Please review the Clinical Evidence section below. Should you have any questions about the data, please contact your Territory Manager.
If you have a question that was not answered here, please contact us.
Clinical Evidence
- Efficacy of a Nebulizer Filter Kit to Prevent Environmental Contamination During Nebulizer Therapy
- A Risk Evaluation for Fugitive Aerosol Emissions When Using Different Nebulizer Systems; Potential Mechanisms, Impact of Device Type, and Mitigation Strategies
- Efficiency of a Nebulizer Filter Kit to Prevent Environmental Contamination During Nebulizer Therapy
References:
1Data on file TMI AL 1872.
2The bacterial and viral filtration efficiency performance of the filtration media has been tested by the material’s manufacturer. Report available upon request.