 Let Breathing Take Control
Let Breathing Take Control
The AEROECLIPSE® II BAN® Nebulizer creates aerosol only in response to the patient’s inspiratory flow.
 Not All Chambers Are Equal
Not All Chambers Are Equal
Some “antistatic” valved holding chambers are only poorly antistatic and are noninterchangeable, which means that switching between them should be discouraged.1
 Improves Patient Outcomes
Improves Patient Outcomes
Recent publications support use of the AEROBIKA® Oscillating Positive Expiratory Pressure device to help reduce readmissions or improve aerosol deposition when used alongside standard of care.2
Recent Poster Presentations
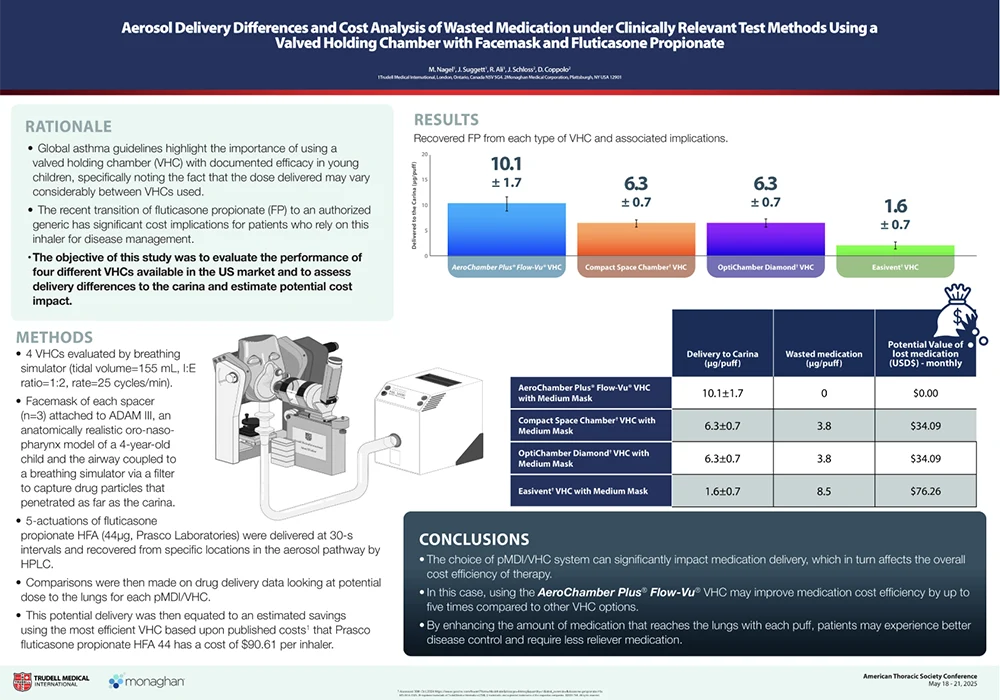
The High Price of Inhaler Waste – ATS 2025
The recent transition of fluticasone propionate (FP) to an authorized generic has significant cost implications for patients who rely on this inhaler for disease management. This poster evaluated four chambers available in the US to assess drug delivery and the cost impacts of these differences. By enhancing the amount of medication that reaches the lungs with each puff, patients may experience better disease control and require less reliever medication. Download the PDF.
Optimizing Delivery of AirSupra in 2 Chamber Varieties – ATS 2025
Our Valved Holding Chambers can be effective in reducing oropharyngeal deposition, facilitating effective lung delivery and lowering the risk of systemic side effects. This poster highlights in vitro lab data supporting how our Valved Holding Chambers can deliver the intended dose while also reducing deposition in the mouth and throat compared to using the inhaler alone. The novel design of a chamber and carrying case in one, may also help improve therapy compliance by keeping the MDI protected while on-the-go. Download the PDF.

AEROECLIPSE® II BAN® Nebulizer
Let Breathing Take Control
The AEROECLIPSE® II BAN® Nebulizer is a leading-edge device designed for efficient medication delivery. It produces aerosol only on inhalation, reducing medication waste and the aerosol particulate that ends up in the room by 3-4 times than that of a continuously run jet nebulizer.3,4 This helps minimize exposure to environmental loss for front line healthcare professionals.
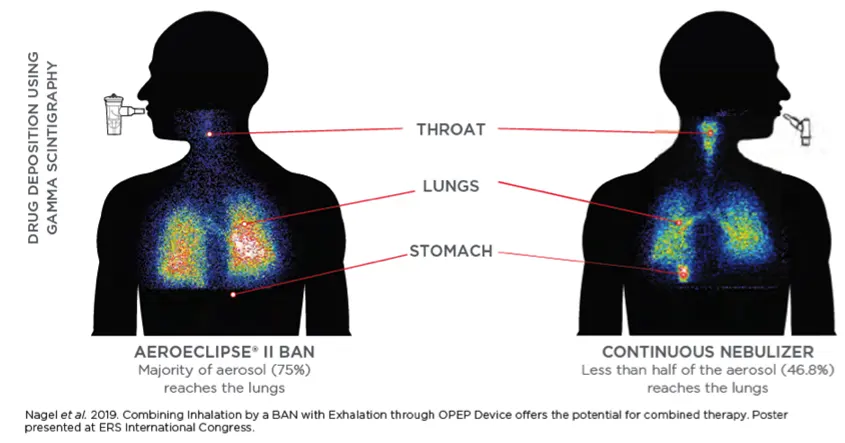
Clinical Evidence
Not all jet nebulizers are created equal. For dose assurance and a safe environment for your frontline staff, choose AEROECLIPSE® II BAN® Nebulizer.
Implementation of the BAN® Nebulizer leads to Savings and Efficiency Gains
The study reports use can significantly reduce hospital admissions and emergency department bed hours for asthma and COPD exacerbations, leading to substantial cost savings and improved operational efficiency. Despite a modest increase in per-unit cost, the investment pays for itself many times over, driven by shorter patient stays and a significant reduction in avoidable admissions. See the full details and calculate the value it could bring your ED here.
Nebulizer Design Contributes to Frontline Safety when Fugitive Emissions are Low
This study reinforces how nebulizer design affects therapeutic dose to the patient and how the AEROECLIPSE® II BAN® Nebulizer can create safer environments by decreasing environmental loss. Learn more.
Implementation of AEROECLIPSE® II BAN® Nebulizer based therapy has the potential to reduce costs associated with staff acquisition of nosocomial influenza
Implementation of the AEROECLIPSE® II BAN® Nebulizer in a hospital Emergency Department resulted in an 88% reduction in the cost of sick days by reducing the number of sick days by 60%. Learn more.
Combining Nebulizer and OPEP Therapy Can Save Time While Delivering Substantially Equivalent Respirable Dose
Use of the AEROECLIPSE® II BAN® Nebulizer alone or with the AEROBIKA® OPEP Device delivers a similar respirable dose. Use of combination therapy saves time without impacting therapeutic benefit.
- Aerosol Delivery Efficiency of the AEROECLIPSE® And PARI LC PLUS® NEBULIZERS with and without Handheld Oscillatory Pressure Therapy.
- Combining Drug Delivery via Breath Actuated Nebulizer with
Exhalation Through An Oscillating Positive Expiratory Pressure Device — The Potential For Optimal Combined Therapy.
If your nebulizer runs continuously, how much is being lost to the room?
This bench study evaluated the environmental loss of 10 commonly used small volume nebulizer using 4 simulated adult breathing patterns. As exhalation time increased, environmental loss increased for all nebulizers except the AEROECLIPSE® II BAN® Nebulizer. These findings could help develop policies and best practices for the risk mitigation of fugitive emissions from nebulizing systems. Learn more.
Full Clinical Study Summary
This Study Summary is designed to identify how the AEROECLIPSE® II BAN™ Nebulizer has performed in both in vitro and in vivo studies with various formulations and versus other nebulizers. Learn more.
AEROCHAMBER PLUS® FLOW-VU® AVHC
For respiratory patients, getting the most from life means getting the most from their inhaler medication.
AEROCHAMBER PLUS® brand of chambers delivers the intended dose established by the pharmaceutical company.⁶ Use of other chambers may lead to medication waste, less than optimal doses, and varying therapeutic responses.
Clinical Evidence
Still think chambers aren’t useful for adults? See what even a ½ second delay can mean to delivered dose
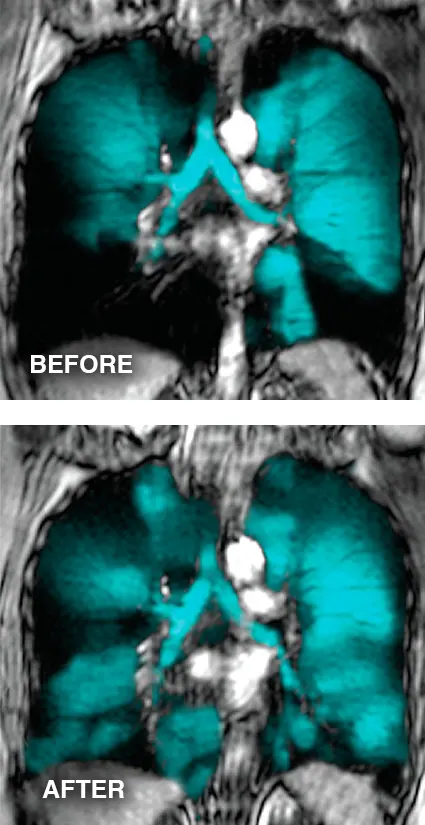
This study uses Functional Respiratory Imaging (FRI) deposition profiles to highlight the significant negative impact on lung deposition of even a relatively short 0.5 second delay between actuation and inhalation when an MDI is used alone. Use of an AEROCHAMBER PLUS® FLOW-VU® aVHC, even with a 2 second delay, demonstrated greater central lung delivery than the MDI alone with perfect coordination reinforcing the importance of chamber use for all people using MDIs. Learn more.
Valved Holding Chambers are not all equivalent in performance and as a result should not be regarded as interchangeable
Only AEROCHAMBER PLUS® FLOW-VU® chambers were equivalent to the reference device data listed in virtually all innovator inhalers currently approved in the US and European markets. Differences in chamber design, materials and function mean that chambers should not be automatically considered interchangeable. Learn more.
Different chambers deliver different amounts of medication which can lead to meaningful differences in clinical performance
The author found that it was unequivocal that differences exist between different chambers which in a number of cases are sufficiently large that meaningful and overt clinical differences would be anticipated as a result. Learn more.
Chamber Design significantly impacts lung deposition and aren’t interchangeable
Peripheral lung deposition was appreciably higher using the AEROCHAMBER PLUS® FLOW-VU® aVHC versus two competitive chambers. These results highlight that Valved Holding Chambers should not be considered interchangeable. Learn more.
Changing patients to DPI medications may result in sub-optimal lung delivery
Inspiratory flow rate is known to influence drug delivery. This study assessed an MDI + chamber and two DPI systems at optimal and sub-optimal flow rates to determine the impact of drug delivery (modelled using Functional Respiratory Imaging). Learn more.
CHAMBER SELECTION IMPACTS THE AMOUNT OF DRUG DELIVERED AND HAS REAL COST IMPLICATIONS TO PATIENTS
The recent transition of fluticasone propionate (FP) to an authorized generic could have aerosol delivery changes that may affect dosing to the lungs and have significant cost implications for patients who rely on this inhaler for disease management. This poster evaluated the different drug delivery profiles of four chambers and assessed the cost impacts of these differences. By enhancing the potential amount of medication that reaches the lungs with each puff, patients may experience better disease control and require less reliever medication. Learn more
This Study Summary is designed to identify how the AEROCHAMBER PLUS® FLOW-VU® Chambers has performed in both in vitro and in vivo studies with various formulations and versus other chambers. Learn more.


AEROBIKA® OPEP Device
Oscillating Positive Expiratory Pressure or OPEP therapy helps stent open and clear blocked airways. On exhalation the device creates a unique oscillation and pressure dynamic to address the structural and functional challenges in the airways. The combination of oscillations and pressure opens the airways and aids in moving mucus into the central airways, where it can be coughed out. The device is drug-free so there are no side effects or drug interactions and it is easy-to-use, so patients are able to transition home with the device.
Clinical Evidence
Significantly Reduce COPD Exacerbations and Treatment Costs in the Critical 30-Day Post-Exacerbation Period
There is growing pressure to improve care and reduce hospital admissions. The AEROBIKA® OPEP device offers both clinical benefits and cost savings by significantly reducing COPD readmissions by 28% in the critical 30 days post-exacerbation.
80% Lower 30-Day Readmission Costs Due to Fewer Readmissions and Shorter Stays from Post-Op Pulmonary Complications
The AEROBIKA® OPEP device is a cost-effective solution for preventing and treating post-operative pulmonary complications. This real world study reports a 39% reduction in all-cause re-hospitalizations, shorter hospital stays, and 80% lower post-discharge costs compared to standard care. Learn more.
Combining OPEP with nebulizer treatments can save time for Bronchiectasis therapy
This study evaluated delivery of hypertonic saline using an AEROECLIPSE® XL R BAN® Nebulizer attached to the AEROBIKA® OPEP device. Use of combination therapy in which neither treatment is diminished, will reduce the burden of care with the benefit of treatment time savings for mucus management in bronchiectasis patients. Learn more.
Still using IS at the bedside? Real World Evidence supports the guideline recommendations to discontinue its use
This real-world study looked at the difference in post-surgical patient outcomes between those given an AEROBIKA® OPEP device versus Incentive Spirometry (IS). The mean number of PPCs per patient with access to devices on day 1 was .57 for OPEP and .77 for IS, highlighting the fact that current US hospital practice favors introducing IS earlier than OPEP for post-operative care. Learn more.
This Study Summary is designed to identify how the AEROBIKA® OPEP Device has performed in in both in vitro and in vivo studies and versus other PEP and OPEP devices. Learn more.
Words or phrases accompanied by ™ and ® are trademarks and registered trademarks of Monaghan Medical Corporation or an affiliate of Monaghan Medical Corporation.
References
1. Hagedoorn P, et al. J Aller Clin Immunol 2020;8(3): 1124-1125e4.
2. Burudpakdee C, et al. Pulmonary Therapy 2017;3(1):163-171; Burudpakdee C, et al. Pulmonary Therapy 2018;4(1):87-101; and Leemans G, et al. COPD 2020;15: 1261–1268.
3. Rau, JL, et al. Performance Comparison of Nebulizer Designs: Constant-Output, Breath-Enhanced, and Dosimetric. Resp Care 2004;(49):174-179 and TMI Aerosol Lab, data on file (600cc, 10BPM, 1:2, Albuterol Sulfate Inhalation Solution 833µg/mL).
4. Nagel M, et al. Am J Respir Crit Care Med 2021;203:A4672.
5. Nagel M, Suggett J, Mitchell JP. RDD 2021;1:287-292.