Access and availability of Albuterol nebulizer solution may be limited due to manufacturing and supply chain issues. This resource aims to provide prescribers with therapeutic options for patients needing a short-acting bronchodilator medication (SABA) until desired concentrations become available. Clinicians should use their expert clinical judgment for conserving albuterol during a shortage in making treatment decisions for each patient.
Considerations for Therapeutic Substitutions:
1. Consider sourcing a different brand of Albuterol
Please see the following links:
Click here for more up to date information on Albuterol Inhalation Shortage.
2. Consider using a different concentration of Albuterol based on the following clinical evidence
- The multi-dose bottles and the 2.5mg/3ml vials may be the most difficult to acquire.
- 0.5% 2.5 mg/0.5ml vials may be available and can be used with additional saline fill in most nebulizers but can also be used without additional fill in the AEROECLIPSE® II BAN® Nebulizer saving clinician time. Because this nebulizer only aerosolizes on inhalation, no medication is lost on exhalation and the concentrated vials give a short and effective therapy.
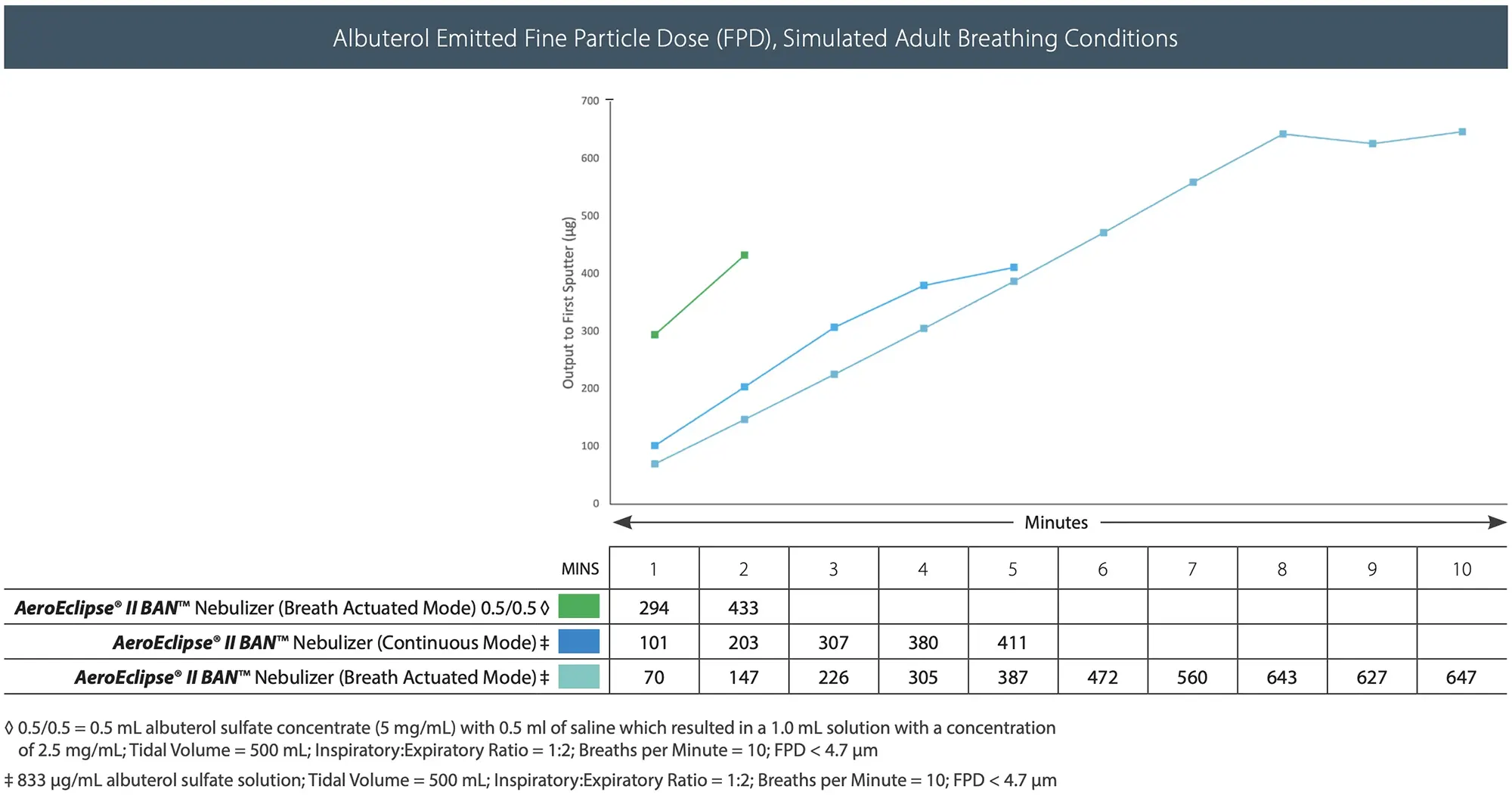
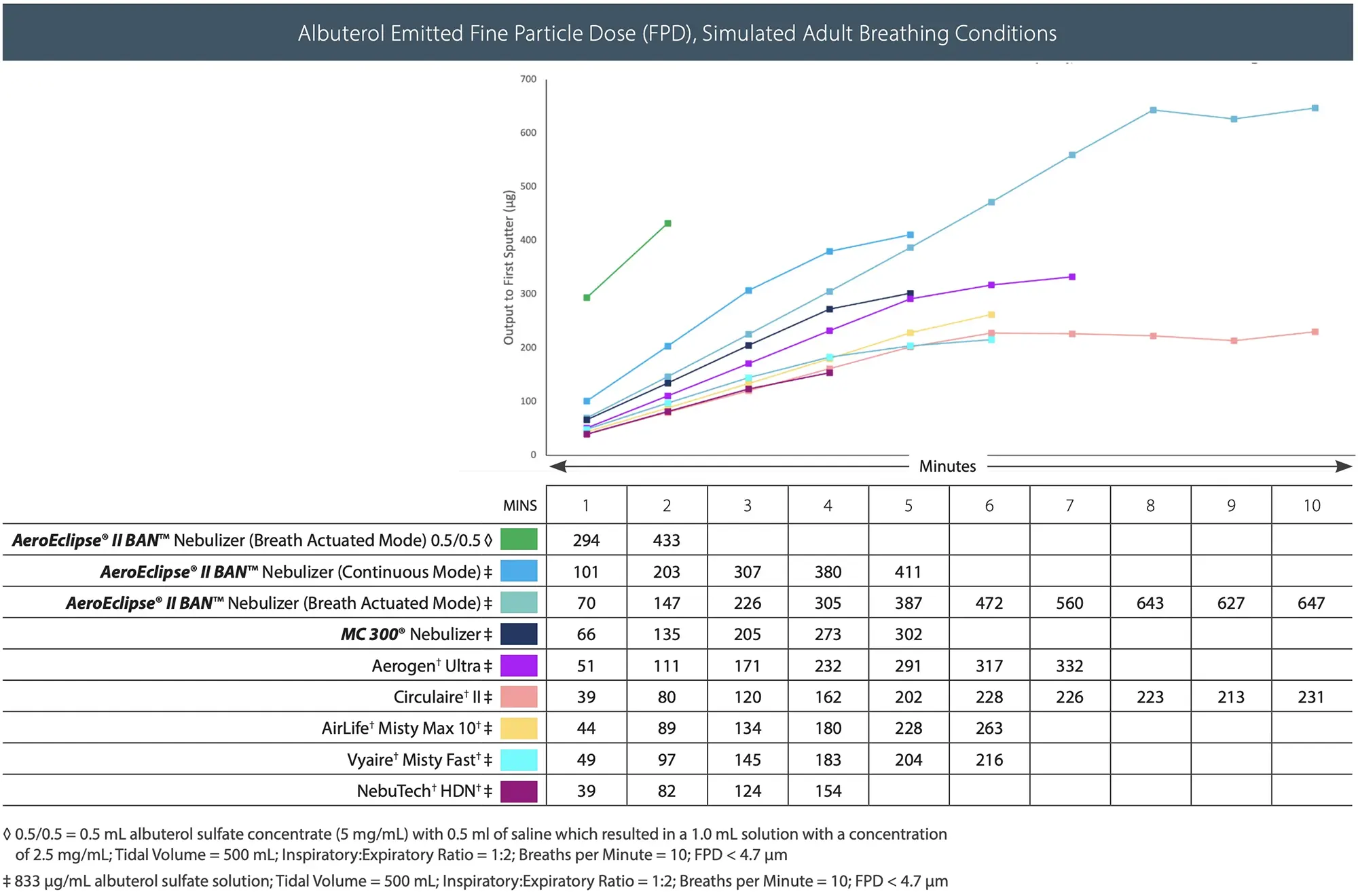
- See graph for medication delivery and duration of therapy for different albuterol concentrations with the AEROECLIPSE® II BAN® Nebulizer.
Evidence
- Reducing Total Costs of Aerosolized Medication Delivery Using the AEROECLIPSE* II Breath Actuated Nebulizer
J Wilson. Respiratory Care 2011;56(10):1634. - Clinical Evaluation of a Breath Actuated Small Volume Nebulizer (BA-SVN)
S Klopf, N Schneiderman, H Payne, C Schramm, MW Nagel, JP Mitchell. Respiratory Care 2000;45(8):979. - The Delivery Time, Efficacy, and Safety of Beta Agonist Bronchodilator Administration with the AEROECLIPSE* Breath Actuated Nebulizer (“BAN”) Versus a Conventional T-Type Small Volume Nebulizer
RS Pikarsky, T Farrell, R Acevedo, W Fascia, C Roman. Respiratory Care 2001;46(10):1085. - The Delivery Time, Efficacy, And Safety Of Β-Agonist Bronchodilator Administration with the AEROECLIPSE* Breath Actuated Nebulizer (“BAN”)
RS Pikarsky, T Farrell, R Acevedo, W Fascia, C Roman. CHEST 2001;120(4):218S. - Rapid Delivery of Bronchodilator Medication Is Possible Using A Breath-Actuated Small Volume Nebulizer as an Alternative To Extended Delivery of Medication By Large Volume Nebulizer
DP Coppolo, JP Mitchell, KJ Wiersema, CC Doyle, MW Nagel. Respiratory Care 2007;52(11):1582.
3. Consider substitution to Lev Albuterol
- Lev albuterol comes in both liquid form for nebulization and in Metered Dose Inhaler (MDI)
- Dosing:
- 1.25 mg lev albuterol = 2.5 mg of albuterol
- 0.63 mg lev albuterol = 1.25 mg of albuterol
Evidence
- Levalbuterol 1 ML (0.42 MG) Q8H Dosing Using The AEROECLIPSE Breath Actuated Nebulizer
Robert S Pikarsky, BSRT, Russell A. Acevedo, MD, FAARC, FCCP, Tracey Farrell, RRT and Wendy Fascia, RRT, Respiratory Care, Crouse Hospital, Syracuse, New York - Improving Resource Utilization with New TechnologiesS
MA Lewis, SS Harris, SL Campbell, AL Hodges, DM Clark. Respiratory Care 2000;45(8):981. - Comparison in Rates of Breakthrough Treatments During a Conversion From Racemic Albuterol to Levalbuterol
RS Pikarsky, RA Acevedo, T Farrell, R Bear, W Fascia. Respiratory Care 2003;48(11):1080. - Safety And Efficacy Of Five-Minute Timed Aerosol Administration With the AEROECLIPSE* Breath Actuated Nebulizer: Comparison of Levalbuterol With Racemic Albuterol
RS Pikarsky, R Acevedo, C Roman, W Fascia, T Farrell. Respiratory Care 2002;47(9):1075.
4. Consider using an Albuterol MDI
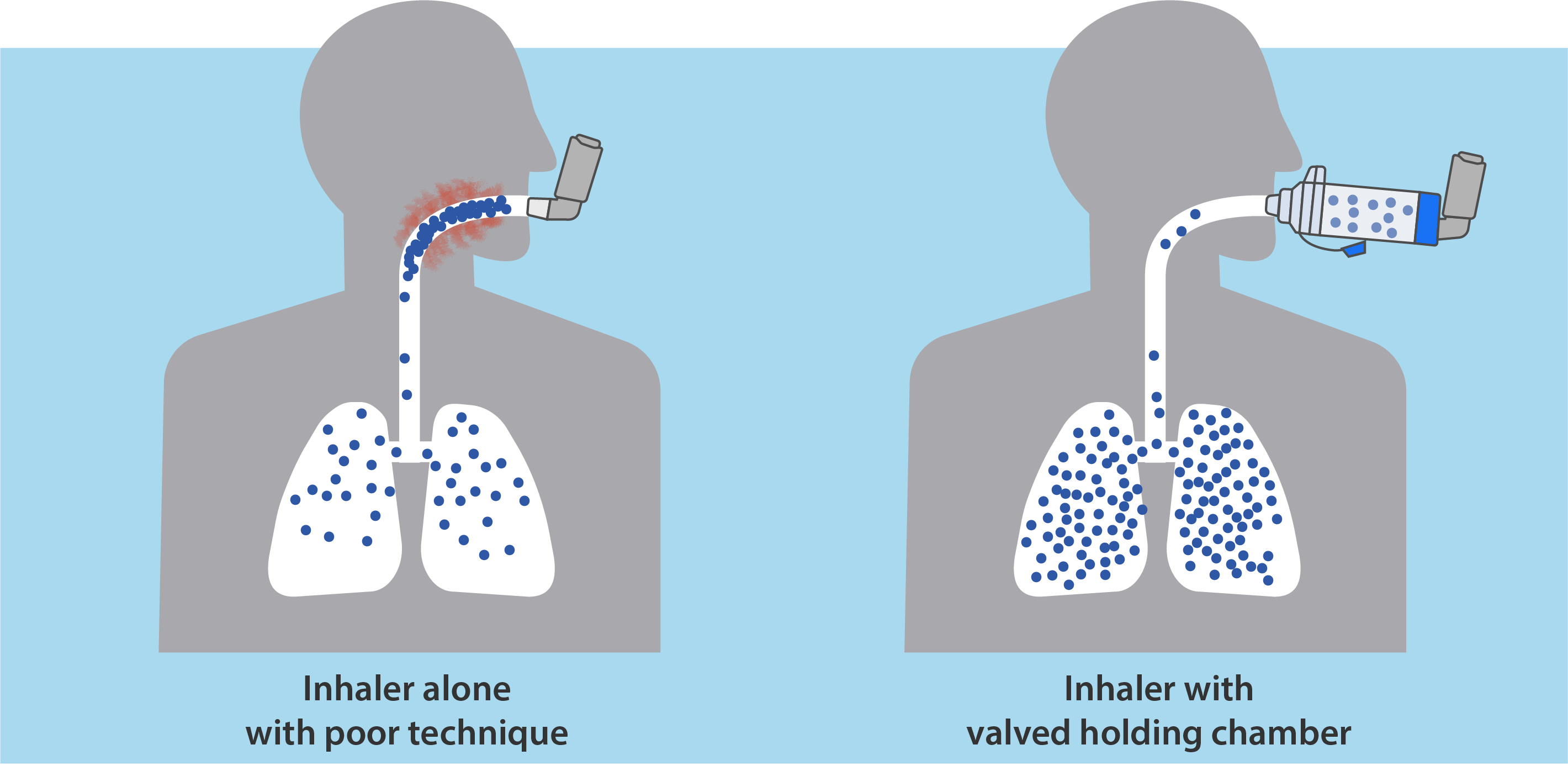
- Substitution to a metered dose inhaler (MDI) is an easy and cost-effective alternative to liquid albuterol. Valved Holding chambers like the AEROCHAMBER PLUS® FLOW-VU® brand of chambers reduce errors associated with the timing of actuation and inhalation, ensuring the intended medication dose is delivered to the patient.1 This brand covers a range of users (infant to adult) who would use either mouthpiece or facemask designs and can accommodate tracheostomy patients. Substitution to MDI’s can also cover ventilated patients with inline devices such as the AEROVENT PLUS® chamber or the AEROCHAMBER® MINI HC.
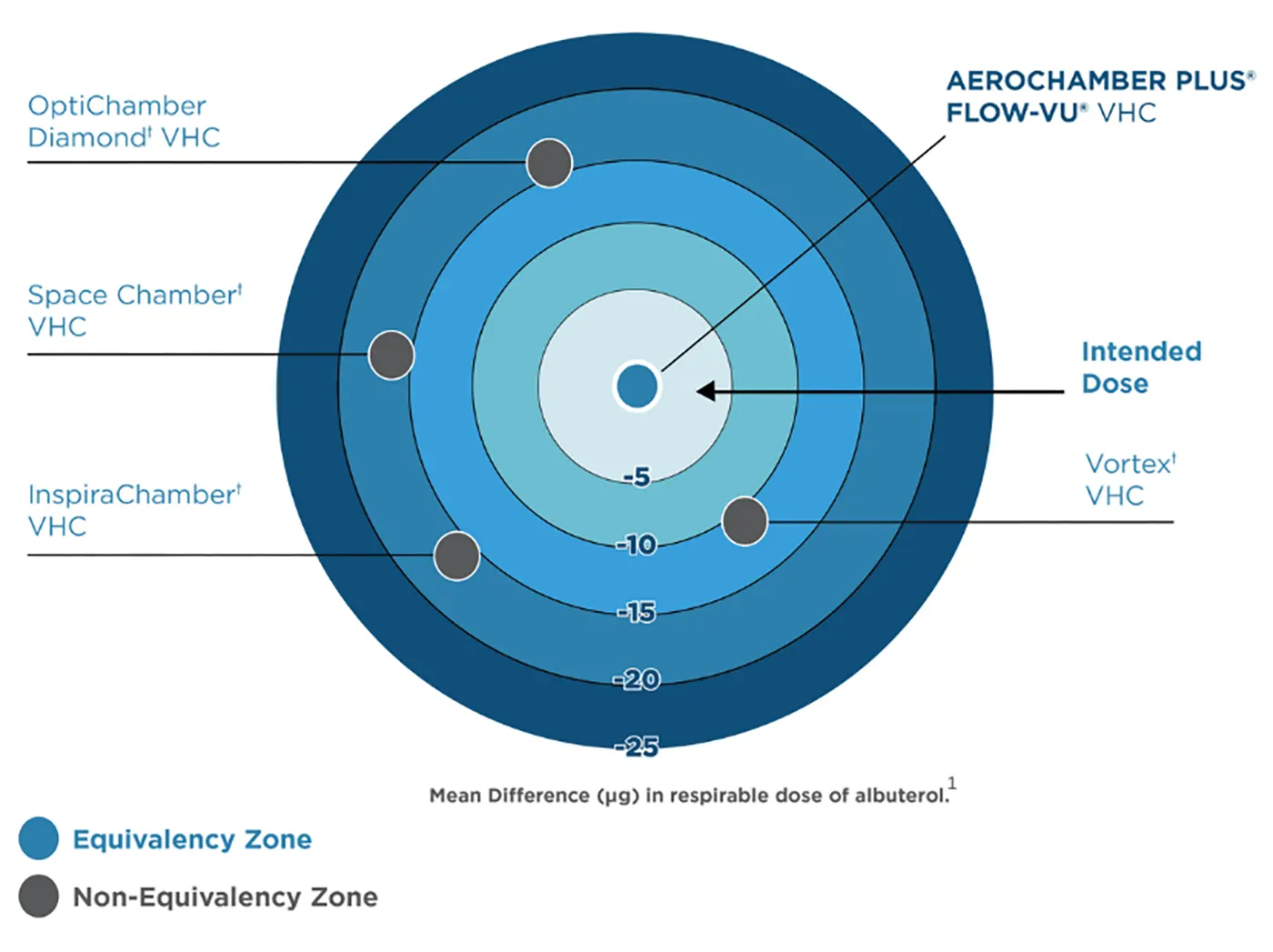
Validate Safety and Efficacy
AEROCHAMBER PLUS® brand of chambers delivers the intended dose established by the pharmaceutical company.1 Other chambers cannot make this claim because they deliver less2,3, meaning patients are wasting expensive medication and may not be receiving the optimal therapeutic dose.
5. Consider conserving medication with the following methods
- Implement therapist driven protocols to ensure the patient receives the right medication at the right time to treat symptoms. These protocols can also be utilized to determine which patients can transition over to an MDI with a valved holding chamber or should continue to receive a nebulizer therapy.
- High Efficiency nebulizers like the AEROECLIPSE® II BAN® Nebulizer get more medication to the symptomatic areas of the lungs (see graph below) and may reduce the need for repeat or frequent nebulizer therapy conserving the amount of liquid albuterol used.4,5,6
- Consider using long-acting bronchodilators (LABA) in the AM/PM to avoid multiple doses throughout the day with short acting agents. These medications come in liquid form and in handheld inhalers (including soft mist inhalers (SMI)).
- If concerns with inhalation and the actuation arise with either MDIs or SMIs, use of a valved holding chamber may improve medication delivery.
- The AEROCHAMBER® FLOW-Vu® chambers are FDA 510K cleared for use with soft mist inhalers and metered dose inhalers
Evidence
- Clinical and Economic Outcomes With a Conversion to Arformoterol Once or Twice Daily From Levalbuterol Using Breath Actuated Nebulizers
RS Pikarsky, RA Acevedo, T Farrell, W Fascia, R Bear. Respiratory Care 2008;53(11):1545.
References:
- Harkness H, et al. CRC 2012.
- Nagel M, et al. CSACI 2017.
- Dissanayake S et al. Pulmonary Pharmacology & Therapeutics 2018;48:179-184.
- Wilson J. Respiratory Care 2011;56(10):1634.
- Titus M, et al. Clinical Pediatrics 2012;51(12):1150-1154.
- Bong C, et al. Ped Academ Soc, Baltimore, MD, 2009.
† trademarks and registered trademarks of the respective companies.
Page updated on March 21, 2023